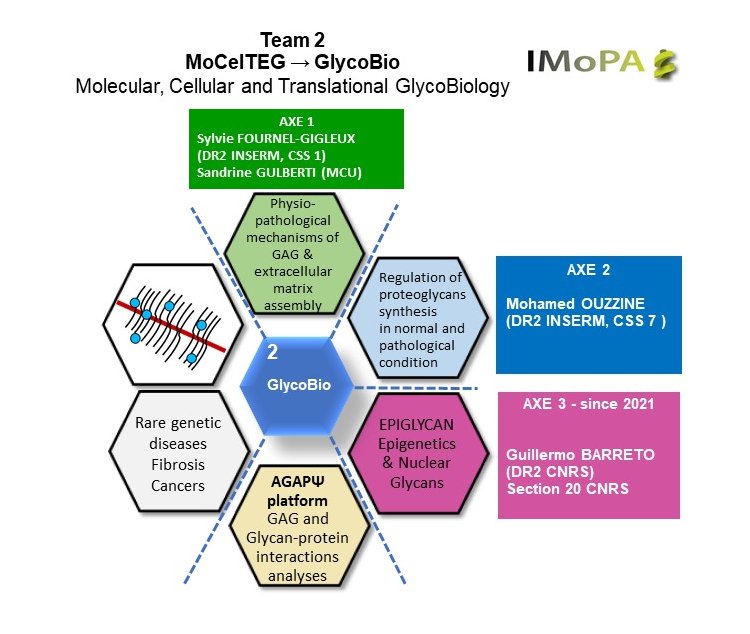
Leaders :
Sylvie Fournel-Gigleux
Mohammed Ouzzine
Guillermo Barreto
GAGs are the most abundant molecules on earth and fundamental components of the cells and tissues of living organisms. Their essential role in a multitude of biological mechanisms, as well as their therapeutic and diagnostic potential, have long been underestimated. These complex biomolecules are sulfated linear oligosaccharide polymers, linked to proteins in the form of proteoglycans (PG). PGs are strategically associated with plasma membranes, abundant in extracellular matrices (ECMs), and recently observed in the cell nucleus. Their significant capacity for interaction with cellular effectors (such as growth factors and cytokines) makes PGs and GAGs fundamental physiopathological regulators of cellular and tissue processes.
The research of GlycoBio team is based on multi-scale approaches, from the molecule to the patient to have fundamental knowledge in glycobiology in order to offer therapeutic and diagnostic tools in pathologies involving disturbances in the biosynthesis of GAGs and/or or collagens. The team explores the biosynthetic machinery and the physiopathological mechanisms of assembly of GAGs and collagens, the main macromolecules of the cellular micro- and macro-environment. We study the structure, functions and regulations of the enzymes responsible for the biosynthesis and maturation of GAGs (glycosyltransferases, sulfotransferases) and collagen (lysyl oxidase, prolyl hydroxylases), but also the atypical molecular functions of GAGs, particularly in the nuclear organization.
Keywords: Proteoglycans (PG), glycosaminoglycans (GAG), extracellular matrix (ECM), collagen, glycosyltransferases, epigenetics, multi-omics approaches, cancer, fibrosis, rare genetic diseases
UPLC-MS
muti-omics
nano-HPLC
mass spectrometry
molecular modelling
FPLC
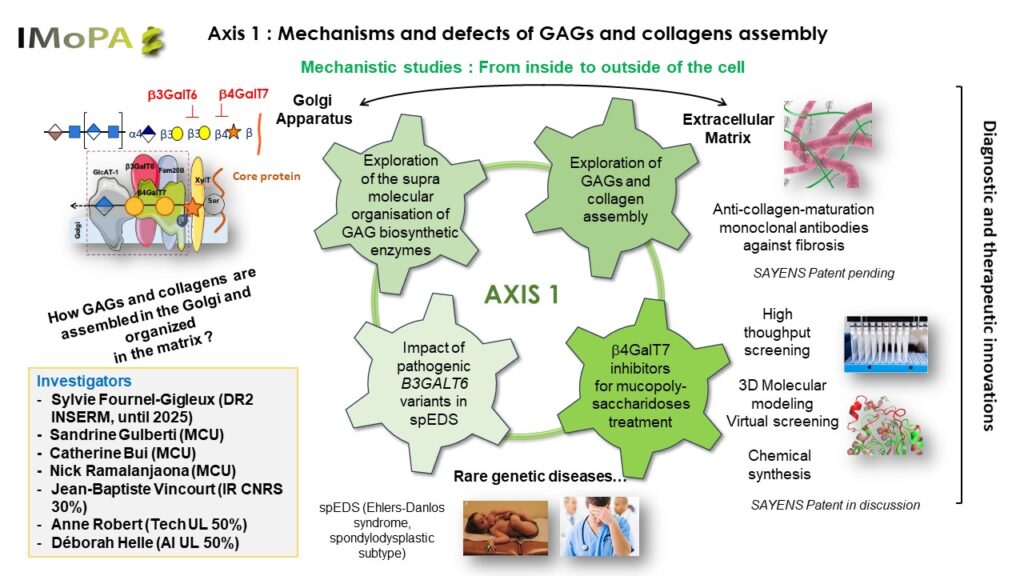
The objective of axis 1 is to understand the assembly mechanisms of GAGs and collagens as well as their interactions in the extracellular matrix in a physiopathological situation. We mainly target rare genetic diseases due to pathogenic variants of GAG biosynthesis initiation enzymes (also called linkeropathies) involved in certain Ehlers-Danlos syndromes (EDS). We also study other rare genetic diseases of GAG metabolism such as mucopolysaccharidoses (MPS) and more common pathologies such as fibrosis and cancers in which GAGs and collagens play an important role. Ultimately, knowledge of these biosynthesis systems and their regulation will make it possible to develop therapies that correct the metabolisms affected in pathological situations.
We explore the hypothesis according to which glycosyltransferases and their potential partners (such as kinases or sulfotransferases) are associated in multiprotein complexes in the Golgi apparatus, ensuring and regulating the formation of the primer of GAG chains. To demonstrate this, we use immunoprecipitation and fluorescence techniques, biophysical and structural approaches (collaboration with IBS in Grenoble) and bioinformatics (collaboration with LORIA in Nancy). We study in silico and experimentally the consequences of pathogenic variants of a glycosyltransferase involved in the biosynthesis of the primer, β3GalT6, on its structure and function and the potential deleterious influence of mutations on the formation of multiprotein complexes (project funded by ANR Glycolink in 2023).
We also investigate several diagnostic avenues for EDS based on transcriptomic and proteomic approaches to achieve a reliable diagnosis of the disease, thus completing the identification of the mutation of the genes encoding the target enzymes of these linkeropathies (collaboration with the Molecular Genetics Center of Gent in Belgium). Complementary biochemical and mass spectrometry approaches will provide additional data on the maturation and assembly of GAGs and collagens. The correction of pathogenic variants by molecular engineering is an avenue that we are exploring in parallel using cellular models invalidated for target genes. We have also initiated the development of GAG biosynthesis inhibitors by targeting an early enzyme in the biosynthesis pathway, β4GalT7 in the context of the treatment of mucopolysaccharidoses (MPS). This project is based on the identification in our team of candidates from high-throughput screening capable of inhibiting the activity of β4GalT7 in vitro (collaboration with PCBIS in Strasbourg). The project includes the validation of drug candidates in vitro and in cellulo as well as in silico approaches using dynamic three-dimensional bioinformatics models. Other enzymes or cofactors/partners could at the same time be considered as targets for inhibitors (project financed by the Rare Disease Foundation).

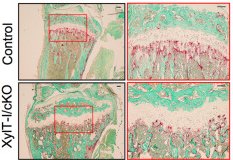

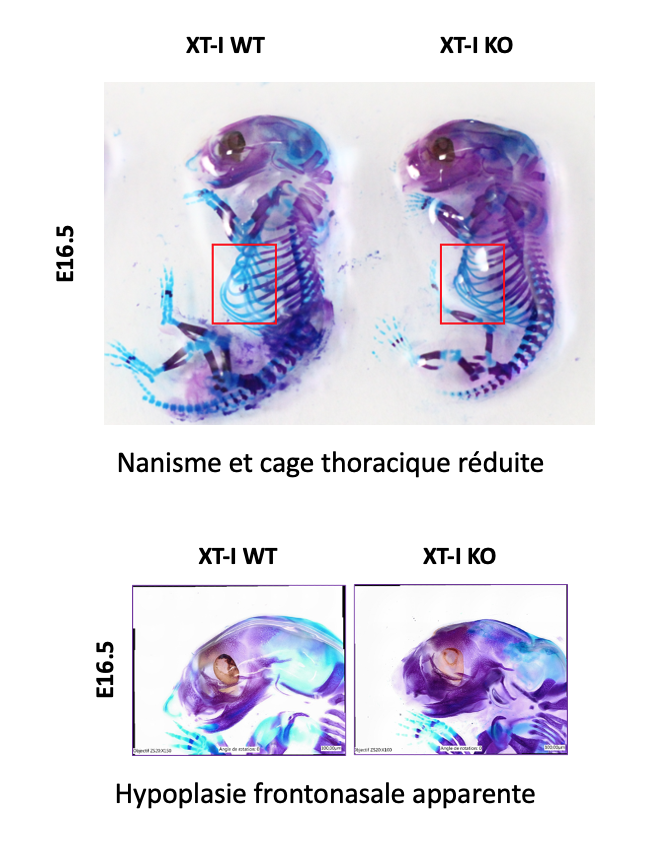
The work of axis 2 focuses on the study of the physiopathological role, the regulation in normal and pathological conditions of proteoglycans (PGs). We developed knockout mice for the XT-I, GT gene responsible for initiating the synthesis of GAG chains of PGs. We have shown that XT-I deficiency in mice induces severe dwarfism due to the acceleration of chondrocyte hypertrophy and endochondral ossification associated with a loss of organization of chondrocytes and collagen fibers in the growth plate. These abnormalities could be the cause of dwarfism produced by XT-I deficiency in patients with Desbuquois syndrome. We then wish to identify the mechanisms responsible for the loss of synthesis of long GAG chains of PG induced by the invalidation of XT-I. Identify the mechanisms responsible for the bone abnormalities observed in XT-I deficient patients using knockout mice for the gene at the level of osteoblast progenitors.
The work also focuses on TMEM165, a Golgi protein identified in patients with CDG syndrome and whose function is believed to be the maintenance of Ca2+/Mn2+ ion homeostasis. We have shown for the first time that TMEM165 deficiency inhibits the polymerization of GAG chains of PG using fibroblasts from CDG patients and cells invalidated for TMEM165. We generated mice knocked out for the TMEM165 gene in cartilage and bone, respectively. These mice present significant dwarfism and bone abnormalities mimicking human pathology. Our objectives are: 1) Determine the impact of TMEM165 deletion on PG synthesis in the growth plate and in bone tissues. 2) Explore the abnormalities of osteoarticular development and determine the molecular mechanisms involved. 3) Evaluate the effect of Mn2+ supplementation on the phenotype of mice invalidated for the TMEM165 gene. This project is funded by the ANR ENIGMncA obtained in 2022.

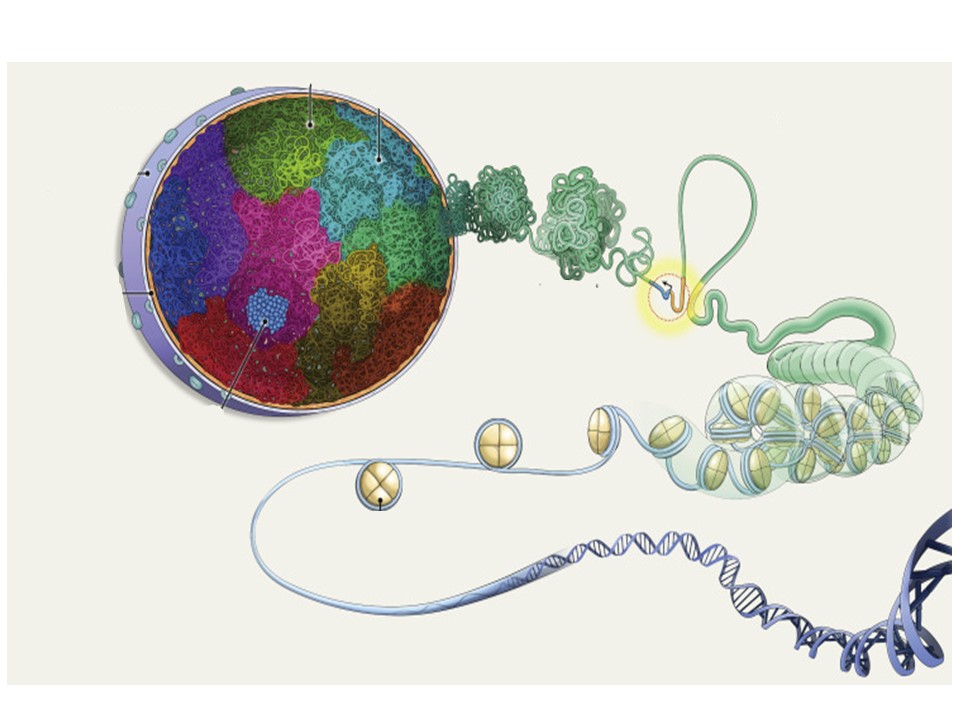
The scientific activities of the research group EPIGLYCAN are centered on the epigenetic mechanisms regulating transcription and the three-dimensional (3D) organization of the genome in physiological conditions and their alterations in chronic pathologies, such as lung cancer and lung fibrosis.
We have extensive scientific expertise in chromatin-mediated transcription regulation and 3D genome organization mediated by macromolecular RNA-protein complexes containing non-histone chromatin-associated proteins, nuclear non-coding RNAs (ncRNAs) and DNA secondary structures. In this context we also study the role of nuclear glycans.
We use in our projects high-resolution mass spectrometry and next generation sequencing (NGS) technologies to monitor various aspects that are relevant for chromatin-mediated transcription regulation and 3D genome organization, such as nuclear proteins, nuclear glycans, histone modifications, histone deposition, DNA methylation, regulatory ncRNAs, secondary structures of nucleic acids, chromosomal territories, self-interacting topologically associating domains (TADs), among others.
The integrative analysis of these multi-omics studies provides an overview of the processes occurring in the nucleus of the cells in physiological conditions and their alterations in chronic pathologies. The mechanisms identified in these multi-omics studies are then confirmed in functional experiments based on in vivo pathological models in mice and on human tissues.




| IE, ULProjets : Biographie Bureau : BiopôleContacts |
| DR, CNRS My research group studies the epigenetic mechanisms regulating transcription and 3D genome organization in physiological conditions and their alterations in chronic pathologies. I have published over 55 articles in recognized scientific journals. I am principal inventor of 2 patents currently licensed, with clinical applications. I am co-directing a clinical study. In my group, I supervised 23 scientists (5 post-docs, 11 PhDs, 7 Masters) who are pursuing promising careers. Guillermo Barreto CV – Pub Med – Google Scholar – ORCID List of 10 selected publications: [10] Corderro J, Swaminathan G, Rogel-Ayala DG, Rubio K, Elsherbiny A, Günther S, Braun T, Dobreva G, and Barreto G. 3D genome organization during TGFB-induced transcription requires nuclear microRNA and G-quadruplexes. BioRxiv; 2023. doi:10.1101/2023.12.22.573061 – Manuscript under revision after peer review at Nat Commun (IF 17.694) [9] Rubio K, Romero-Olmedo AJ, Pouya S, Swaminathan G, Ranvir VP, Rogel-Ayala DG, Cordero J, Günther S, Metha A, Bassaly B, Braubach P, Wygrecka M, Gattenlöhner S, Tresch A, Braun T, Dobreva G, Rivera MN, Singh I, Graumann J and Barreto G. Non-canonical integrin signaling activates EGFR and RAS-MAPK-ERK signaling in small cell lung cancer. Theranostics; 2023; 13(8):2319-2342. doi:10.7150/thno.79493 (IF 12.400) [8] Dobersch S, Rubio K, Singh I, Günther S, Graumann J, Cordero J, Castillo-Negrete R, Huynh MB, Mehta A, Braubach P, Cabrera-Fuentes H, Bernhagen J, Chao CM, Bellusci S, Günther A, Preissner KT, Dobreva G, Wygrecka M, Braun T, Papy-Garcia D and Barreto G. Positioning of nucleosomes containing γ-H2AX precedes active DNA demethylation and transcription initiation. Nat Commun; 2021; Feb 16;12(1):1072. doi: 10.1038/s41467-021-21227-y (IF 17.694) [7] Rubio K, Singh I, Dobersch S, Sarvari P, Günther S, Cordero J, Mehta A, Wujak L, Cabreras-Fuentes H, Chao CH, Bellusci S, Günther A, Dobreva G, Wygrecka M, Seeger W, Preissner KT, Savai R, Papy-Garcia D, Heikenwalder M, Pullamsetti SS, Braun T and Barreto G. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in Idiopathic Pulmonary Fibrosis. Nat Commun; 2019; May 20;10(1):2229. doi: 10.1038/s41467-019-10066-7 (IF 17.694) [6] Gao R, Liang X, Cheedipudi S, Cordero J, Jiang X, Zhang Q, Caputo L, Günther S, Kuenne C, Ren Y, Bhattacharya S, Yuan X, Barreto G, Chen Y, Braun T, Evans SM, Sun Y and Dobreva G. Pioneering function of Isl1 in epigenetic control of cardiomyocyte cell fate. Cell Research; 2019; Apr 25. doi: 10.1038/s41422-019-0168-1 (IF 46.297) [5] Jia Y, Vong JS, Asafova AS, Garvalov BK, Caputo L, Cordero J, Boettger T, Günther S, Fink L, Acker T, Barreto G, Seeger W, Braun T, Savai R and Dobreva G. Lamin B1 loss drives lung cancer development and metastasis by epigenetic derepression of RET proto-oncogene. J Exp Med; 2019; Apr 23. pii: jem.20181394. doi: 10.1084/jem.20181394 (IF 17.579) [4] Dobersch S, Rubio K and Barreto G. Pioneer factors and architectural proteins mediating embryonic expression signatures in cancer. Trends Mol Med; 2019; Feb 19. pii: S1471-4914(19)30019-X. doi: 10.1016/j.molmed.2019.01.008 (IF 15.272) [3] Singh I, Contreras A, Cordero J, Rubio K, Dobersch S, Günther S, Jeratsch S, Mehta A, Krüger M, Graumann J, Seeger W, Dobreva G, Braun T and Barreto G. MiCEE is a ncRNA-protein complex that mediates epigenetic silencing and nucleolus organization. Nat Genet; 2018; Jul; 50(7):990-1001. doi: 10.1038/s41588-018-0139-3. Epub 2018 Jun 4. (IF 41.379) [2] Mehta A, Cordero J, Dobersch S, Romero-Olmedo AJ, Savai R, Bodner J, Chao CM, Fink L, Guzmán-Díaz E, Singh I, Dobreva G, Rapp UR, Günther S, Ilinskaya ON, Bellusci S, Dammann RH, Braun T, Seeger W, Gattenlöhner S, Tresch A, Günther A and Barreto G. Non-invasive lung cancer diagnosis by detection of GATA6 and NKX2-1 isoforms in exhaled breath condensate. EMBO Mol Med; 2016; 8, 1380-1389, doi:10.15252/emmm.201606382 (IF 14.000) [1] Singh I, Ozturk N, Mehta A, Cordero J, Hasan D, Cosentino C, Sebastian C, Krüger M, Looso M, Carraro G, Bellusci S, Seeger W, Braun T, Mostoslavsky R and Barreto G. High Mobility Group protein mediated transcription requires DNA damage marker γ-H2AX. Cell Research; 2015 Jul;25(7):837-50; doi: 10.1038/cr.2015.67 (IF 46.297) Bureau : BiopôleContacts |
| MCU, ULProjets : Biographie Bureau : BiopôleContacts |
Ph | DR, INSERMResearch and Teaching My research career is devoted to exploring the structure-function and regulation of glycosyltransferases involved in key biological processes. Since 2000, my team investigates the enzyme machinery responsible for glycosaminoglycan (GAG) synthesis and its pathological disorders in connective tissue disorders and cancer. Our current interest is on the patho-mechanisms of GAG metabolism-related defects and the search for therapeutic and engineering strategies. I have published 121 original articles and reviews (WoS h38 5,047 citations), one patent, edited 2 books and written 4 book chapters. I have trained 15 PhD and > 20 Master students, 9 post-doctoral researchers and 3 technical staff. Since 2000, I have coordinated or co-coordinated > 40 grants. Key-words: structural glycobiology, molecular engineering, biochemistry, physiopathology, glycosyltransferases, glycosaminoglycans, rare genetic diseases, cancer. Biography 1986 – PhD Thesis in Pharmaceutical Sciences, Fce 1987 – 1988 Welcome Trust Post-Doctoral Fellowship, Dundee, UK 1995 –1996 MRC Senior Scientist Fellowship, Dundee, UK 1989 – 2000 Chargée de Recherches INSERM 2009 – 2012 Contrat d’interface INSERM-University of Dundee, UK Since 2000 – Director of Research INSERM Awards 2007 – 2013 Awarded Honorary Professor of the University of Dundee, UK Responsibilities Since 2013 – Co-Head of MolCelTEG Team (Team 2 IMoPA) Since 2013 – Member of Alliance Nationale pour les Sciences de la Vie et de la Santé ITMO BMSV Since 2015 – Member of International Ehlers-Danlos Syndrome Consortium 2010 – 2017 Director of the International Associated Laboratory (IAL SFGEN) CNRS-UL-University of Dundee 2012 – 2017 Member of Scientific Committee “Ecole Doctorale” BioSE 2017 – 2021 Member of CNRS Committee (Comité National CNRS, CoNRS Section 20) Bureau : Biopôle, 2ème étage, 2G38 |
| MCU, ULProjets : Biographie Bureau : BiopôleContacts |
| IE, ULProjets : Biographie Bureau : BiopôleContacts |
Photo | DR, INSERMProjets : Biographie Bureau : BiopôleContacts |
Photo | MCY, ULProjets : Biographie Bureau : BiopôleContacts |
TE, ULProjets : Biographie Bureau : BiopôleContacts |
 | PhD studentI am a PhD student currently in my third year of doctoral studies. I have the privilege of being mentored by Dr. Guillermo Barreto, group leader of EPIGLYCAN international lab, and by Dr. Thomas Braun, director of the Max Planck Institute for Heart and Lung research. Under their guidance, our research aims to characterize the intricate epigenetic mechanisms underlying the development of lung diseases. Diana G. Rogel Ayala CV – Pub Med – Google Scholar – ORCID List of publications: [7] Swaminathan G, Rogel-Ayala DG, Armich A and Barreto G. Implications in Cancer of Nuclear Micro RNAs, Long Non-Coding RNAs, and Circular RNAs Bound by PRC2 and FUS. Cancers; 2024, 16(5), 868; https://doi.org/10.3390/cancers16050868 [6] Cordero J, Swaminathan G, Rogel-Ayala DG, Rubio K, Elsherbiny A, Günther S, Braun T, Dobreva G, and Barreto G. 3D genome organization during TGFB-induced transcription requires nuclear microRNA and G-quadruplexes. BioRxiv; 2023. doi:10.1101/2023.12.22.573061 – Manuscript under revision after peer review at Nature Communications [5] Rubio K, Romero-Olmedo AJ, Pouya S, Swaminathan G, Ranvir VP, Rogel-Ayala DG, Cordero J, Günther S, Metha A, Bassaly B, Braubach P, Wygrecka M, Gattenlöhner S, Tresch A, Braun T, Dobreva G, Rivera MN, Singh I, Graumann J and Barreto G. Non-canonical integrin signaling activates EGFR and RAS-MAPK-ERK signaling in small cell lung cancer. Theranostics; 2023; 13(8):2319-2342. doi:10.7150/thno.79493 [4] Rogel-Ayala DG, Muñoz-Medina JE, Vicente-Juárez VD, Grether-González P, Morales-Barquet DA, Martínez-García AJ, Echaniz-Avliles MOL, Sevilla-Montoya R, Martínez-Juárez A, Arteaga-Vázquez J, Ángeles-Martínez J, Vargas-Alarcón G, Hidalgo-Bravo A and Monroy-Muñoz IE. Association of the EPAS1 rs7557402 polymorphism with hemodynamical significant PDA closure failure in premature newborns under Pharmacological treatment with ibuprofen. Diagnostics (Basel); 2023 Aug 1;13(15):2558. doi: 10.3390/diagnostics13152558. [3] Rubio K, Hernández-Cruz EY, Rogel-Ayala DG, Sarvari P, Isidoro C, Barreto G and Pedraza-Chaverri J. Nutriepigenomics in Environmental-Associated Oxidative Stress. Antioxidants (Basel). 2023 Mar 21;12(3):771. doi: 10.3390/antiox12030771. [2] Aguinaga-Ríos M, Valdespino-Vázquez MY, Medina-Castro D, Espino-Sosa S, Sevilla-Montolla R, Miranda-Araujo O, Acevedo-Gallegos S, Monroy-Muñoz IE, Helguera-Reppeto AC, Pérez-Durán J, Mariscal-Mendizabal LF, Franco-Murillo MR, Lara-Enríquez RM, Armijos-Torres JC, Rogel-Ayala DG, Cardona-Pérez JA. Causal analysis of fetal death in high-risk pregnancies. Journal of Perinatal Medicine; 2021; 49(6), 740-747. https://doi.org/10.1515/jpm-2020-0352 [1] Morales-Avila E, Ferro-Flores G, Ocampo-García BE, López-Téllez G, López-Ortega J, Rogel-Ayala DG, Sánchez-Padilla D. Antibacterial Efficacy of Gold and Silver Nanoparticles Functionalized with the Ubiquicidin (29–41) Antimicrobial Peptide. Journal of Nanomaterials; 2017; vol. 2017, Article ID 5831959. https://doi.org/10.1155/2017/5831959 Bureau : BiopôleContacts |
PhD studentI am a 3rd year Ph.D. student working under the supervision of Dr. Guillermo Barreto. My project focuses on studying different aspects of chromatin structure including three-dimensional genome, non-coding RNAs, histone modifications, and noncanonical secondary DNA structures such as R-loops and G4s in lung adenocarcinoma cells. This project will provide a molecular basis for designing novel therapies for non-small cell lung cancer (NSCLC). Guruprasadh Swaminathan CV – PubMed – Google Scholar – ORCID List of publications: [8] Swaminathan G, Rogel-Ayala DG, Armich A and Barreto G. Implications in Cancer of Nuclear Micro RNAs, Long Non-Coding RNAs, and Circular RNAs Bound by PRC2 and FUS. Cancers. 2024; 16(5):868. doi.org/10.3390/cancers16050868 (IF 5.2) [7] Cordero J, Swaminathan G, Rogel-Ayala DG, Rubio K, Elsherbiny A, Günther S, Braun T, Dobreva G, and Barreto G. 3D genome organization during TGFB-induced transcription requires nuclear microRNA and G-quadruplexes. BioRxiv; 2023. doi:10.1101/2023.12.22.573061 – Manuscript under revision after peer review at Nat Commun (IF 17.694) [6] Rubio K, Romero-Olmedo AJ, Pouya S, Swaminathan G, Ranvir VP, Rogel-Ayala DG, Cordero J, Günther S, Metha A, Bassaly B, Braubach P, Wygrecka M, Gattenlöhner S, Tresch A, Braun T, Dobreva G, Rivera MN, Singh I, Graumann J and Barreto G. Non-canonical integrin signaling activates EGFR and RAS-MAPK-ERK signaling in small cell lung cancer. Theranostics; 2023; 13(8):2319-2342. doi:10.7150/thno.79493 (IF 12.400) [5] Kourani K, Jain P, Kumar A, Jangid AK, Swaminathan G, Durgempudi VR, Jose J, Reddy R, Pooja D, Kulhari H, Kumar L. Inulin coated Mn3O4 nanocuboids coupled with RNA interference reverse intestinal tumorigenesis in Apc knockout murine colon cancer models. Nanomedicine: Nanotechnology, Biology and Medicine; 2022; Feb;40:102504. doi: 10.1016/j.nano.2021.102504. (IF 5.4) [4] Kumar A, Singam A, Swaminathan G, Killi N, Tangudu NK, Jose J, Gundloori Vn R, Dinesh Kumar L. Combinatorial therapy using RNAi and curcumin nano-architectures regresses tumors in breast and colon cancer models. Nanoscale. 2022 Jan 6;14(2):492-505. doi: 10.1039/d1nr04411g. (IF 8.307) [3] Swaminathan G, Shigna A, Kumar A, Byroju VV, Durgempudi VR, Dinesh Kumar L. RNA interference and Nanotechnology: A promising alliance for next generation cancer therapeutics. Front. Nanotechnol. 2021 Jun 9;3:42. 3:694838. doi: 10.3389/fnano.2021.694838 [2] Swaminathan G, Byroju V.V Kumar, A Kourani K, Dinesh Kumar L. (2021). Acute Lymphoblastic Leukemia: Promising Technologies for the Management of the Disease. In L.T. Duncan (Ed.). Advances in Health and Disease. Volume 37 (pp. 1-70). Nova Science Publishers (https://novapublishers.com/shop/advances-in-health-and-disease-volume-37/) -Book chapter [1] Rawoof A, Swaminathan G, Tiwari S, Nair RA, Dinesh Kumar L. LeukmiR: a database for miRNAs and their targets in acute lymphoblastic leukemia. Database (Oxford). 2020 Jan 1;2020:baz151. doi: 10.1093/database/baz151. (IF 5.8) Bureau : BiopôleContacts |
| Research Engineer, HDR Manager IBSLor Proteomics Platform, SMP of University of Lorraine Research: Molecular mechanisms of collagen assembly New therapeutic avenues against fibrotic degeneration Approaches: Tissue and molecular fractionation; relative quantitative analysis without labeling by mass spectrometry; functional characterization of enzymes catalyzing collagen maturation by genetic invalidation in cellular models. Biography: 2002: PhD in cell biology at Toulouse III University. 2010: CNRS platform IR. 2022: HDR from the University of Lorraine. |










Implications in Cancer of Nuclear Micro RNAs, Long Non-Coding RNAs, and Circular RNAs Bound by PRC2 and FUS. Swaminathan G, Rogel-Ayala DG, Armich A, Barreto G. Cancers (Basel). 2024 Feb 21;16(5):868. doi: 10.3390/cancers16050868. PMID: 38473229
Preliminary results from the EMoLung clinical study showing early lung cancer detection by the LC score. Rubio K, Müller JM, Mehta A, Watermann I, Olchers T, Koch I, Wessels S, Schneider MA, Araujo-Ramos T, Singh I, Kugler C, Stoleriu MG, Kriegsmann M, Eichhorn M, Muley T, Merkel OM, Braun T, Ammerpohl O, Reck M, Tresch A, Barreto G. Discov Oncol. 2023 Oct 3;14(1):181. doi: 10.1007/s12672-023-00799-9. PMID: 37787775
F-actin nanostructures rearrangements and regulation are essential for SARS-CoV-2 particle production in host pulmonary cells. Swain J, Merida P, Rubio K, Bracquemond D, Neyret A, Aguilar-Ordoñez I, Günther S, Barreto G, Muriaux D. iScience. 2023 Jul 17;26(8):107384. doi: 10.1016/j.isci.2023.107384. eCollection 2023 Aug 18. PMID: 37564698
Xylosyltransferase I mediates the synthesis of proteoglycans with long glycosaminoglycan chains and controls chondrocyte hypertrophy and collagen fibers organization of in the growth plate. Taieb M, Ghannoum D, Barré L, Ouzzine M. Cell Death Dis. 2023 Jun 9;14(6):355. doi: 10.1038/s41419-023-05875-0. PMID: 37296099
Non-canonical integrin signaling activates EGFR and RAS-MAPK-ERK signaling in small cell lung cancer. Rubio K, Romero-Olmedo AJ, Sarvari P, Swaminathan G, Ranvir VP, Rogel-Ayala DG, Cordero J, Günther S, Mehta A, Bassaly B, Braubach P, Wygrecka M, Gattenlöhner S, Tresch A, Braun T, Dobreva G, Rivera MN, Singh I, Graumann J, Barreto G. Theranostics. 2023 Apr 17;13(8):2384-2407. doi: 10.7150/thno.79493. eCollection 2023. PMID: 37215577
Nutriepigenomics in Environmental-Associated Oxidative Stress. Rubio K, Hernández-Cruz EY, Rogel-Ayala DG, Sarvari P, Isidoro C, Barreto G, Pedraza-Chaverri J. Antioxidants (Basel). 2023 Mar 21;12(3):771. doi: 10.3390/antiox12030771. PMID: 36979019
Tissue-specific collagen hydroxylation at GEP/GDP triplets mediated by P4HA2. Wilhelm D, Wurtz A, Abouelfarah H, Sanchez G, Bui C, Vincourt JB. Matrix Biol. 2023 May;119:141-153. doi: 10.1016/j.matbio.2023.03.009. Epub 2023 Mar 30. PMID: 37003347
Editorial: Molecular basis of epigenetic regulation in cancer therapies. Carlos-Reyes A, Romero-Garcia S, López-Camarillo C, Barreto G, Prado-Garcia H. Front Genet. 2023 Jan 10;13:1115353. doi: 10.3389/fgene.2022.1115353. eCollection 2022. PMID: 36704341
Circulating hyaluronic acid signature in CAP and ARDS – the role of pneumolysin in hyaluronic acid shedding. Sauer A, Seeliger B, Jandl K, Erfinanda L, Wilhelm J, Alexopoulos I, Baal N, Birnhuber A, David S, Welte T, Barreto G, Gaertner U, Kwapiszewska G, Seeger W, Kuebler WM, Schaefer L, Wygrecka M; members of the CAPNETZ Study Group. Matrix Biol. 2022 Dec;114:67-83. doi: 10.1016/j.matbio.2022.11.003. Epub 2022 Nov 16. PMID: 36456058
Alterations in glycosaminoglycan biosynthesis associated with the Ehlers-Danlos syndromes. Syx D, Delbaere S, Bui C, De Clercq A, Larson G, Mizumoto S, Kosho T, Fournel-Gigleux S, Malfait F. Am J Physiol Cell Physiol. 2022 Dec 1;323(6):C1843-C1859. doi: 10.1152/ajpcell.00127.2022. Epub 2022 Aug 22. PMID: 35993517
ADAR1 Isoforms Regulate Let-7d Processing in Idiopathic Pulmonary Fibrosis. Díaz-Piña G, Rubio K, Ordoñez-Razo RM, Barreto G, Montes E, Becerril C, Salgado A, Cabrera-Fuentes H, Aquino-Galvez A, Carlos-Reyes A, Ruiz V. Int J Mol Sci. 2022 Aug 12;23(16):9028. doi: 10.3390/ijms23169028. PMID: 36012303.
Impact of the Exposome on the Epigenome in Inflammatory Bowel Disease Patients and Animal Models. Vieujean S, Caron B, Haghnejad V, Jouzeau JY, Netter P, Heba AC, Ndiaye NC, Moulin D, Barreto G, Danese S, Peyrin-Biroulet L. Int J Mol Sci. 2022 Jul 9;23(14):7611. doi: 10.3390/ijms23147611. PMID: 35886959
Characterization of an Innovative Biomaterial Derived From Human Wharton’s Jelly as a New Promising Coating for Tissue Engineering Applications. Fayon A, Helle D, Francius G, Vincourt JB, Regnault V, Dumas D, Menu P, El Omar R. Front Bioeng Biotechnol. 2022 Jun 13;10:884069. doi: 10.3389/fbioe.2022.884069. eCollection 2022. PMID: 35769101
Differential Effects of D-Galactose Supplementation on Golgi Glycosylation Defects in TMEM165 Deficiency. Durin Z, Houdou M, Morelle W, Barré L, Layotte A, Legrand D, Ouzzine M, Foulquier F. Front Cell Dev Biol. 2022 May 26;10:903953. doi: 10.3389/fcell.2022.903953. eCollection 20
Fgfr2b signaling is essential for the maintenance of the alveolar epithelial type 2 lineage during lung homeostasis in mice. Ahmadvand N, Lingampally A, Khosravi F, Vazquez-Armendariz AI, Rivetti S, Jones MR, Wilhelm J, Herold S, Barreto G, Koepke J, Samakovlis C, Carraro G, Zhang JS, Al Alam D, Bellusci S. Cell Mol Life Sci. 2022 May 19;79(6):302. doi: 10.1007/s00018-022-04327-w. PMID: 35587837
Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs. Olmedo-Suárez MÁ, Ramírez-Díaz I, Pérez-González A, Molina-Herrera A, Coral-García MÁ, Lobato S, Sarvari P, Barreto G, Rubio K. Biomolecules. 2022 Mar 28;12(4):513. doi: 10.3390/biom12040513. PMID: 35454102
Lymphoid-specific helicase in epigenetics, DNA repair and cancer. Chen X, Li Y, Rubio K, Deng B, Li Y, Tang Q, Mao C, Liu S, Xiao D, Barreto G, Tao Y. Br J Cancer. 2022 Feb;126(2):165-173. doi: 10.1038/s41416-021-01543-2. Epub 2021 Sep 7. PMID: 34493821
© 2024 Ingénierie Moléculaire Cellulaire & Physiopathologie. Built using WordPress and the Mesmerize theme